Welcome to Monaghan Medical Corporation
Thank You

Let Breathing
Take Control
The AEROECLIPSE® II Breath Actuated Nebulizer creates aerosol only in response to the patient’s inspiratory flow.

Not All Chambers
Are Equal
Some “antistatic” valved holding chambers are only poorly antistatic and are noninterchangeable, which means that switching between them should be discouraged.¹

Clinically Supported to Improve
Patient Outcomes
Recent publications support use of the AEROBIKA® Oscillating Positive Expiratory Pressure device to help reduce readmissions or improve aerosol deposition when used alongside standard of care. 2
ATS Posters

AeroEclipse® II BAN
Let Breathing Take Control
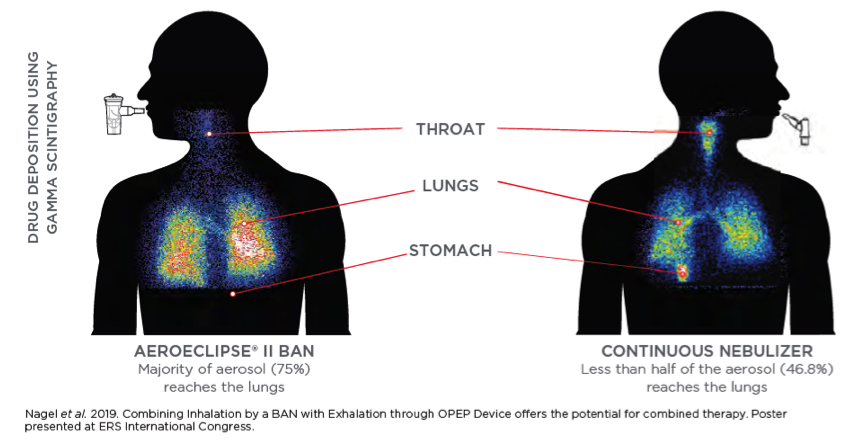
The AEROECLIPSE® II Breath Actuated Nebulizer (BAN) produces aerosol with a high respirable dose and optimal particle size ONLY when the patient is inhaling. Reducing medication waste and the aerosol particulate that ends up in the room by 3-4 times than that of a continuously run jet nebulizer.3,4 This helps minimize exposure to environmental loss for front line healthcare professionals.
Clinical Evidence
Not all jet nebulizers are created equal. For dose assurance and a safe environment for your frontline staff, choose AEROECLIPSE® II BAN.
Significantly more aerosol is delivered to the therapeutically important areas of the lungs when compared to a continuously running nebulizer. Learn more.
When different inspiratory:expiratory ratios were considered, there was significant variance in the Delivered Dose and the Respirable Dose between different brands of non-breath-activated nebulizer. Consideration should be given to revision of the test protocols included in the guidelines, to reflect more accurately the potential therapeutic dose that is delivered to a realistic spectrum of breathing patterns. Learn more.
This Study Summary is designed to identify how the AEROECLIPSE® II Breath Actuated Nebulizer (BAN) has performed in both in vitro and in vivo studies with various formulations and versus other nebulizers. Learn more.
AEROCHAMBER PLUS® FLOW-VU®
For respiratory patients, getting the most from life means getting the most from their inhaler medication.
Clinical Evidence
A quick view of the most recent peer-reviewed evidence supporting the chambers. Learn more.
Only AEROCHAMBER PLUS® FLOW-VU® chambers were equivalent to the reference device data listed in virtually all innovator inhalers currently approved in the US and European markets. Differences in chamber design, materials and function mean that chambers should not be automatically considered interchangeable. Learn more.
The author found that it was unequivocal that differences exist between different chambers which in a number of cases are sufficiently large that meaningful and overt clinical differences would be anticipated as a result. Learn more.
Some “antistatic” valved holding chambers (aVHC) have poor antistatic properties and should therefore be considered non-antistatic and primed. Differences in antistatic properties are an important cause of the large performance differences between aVHCs. Learn more.
For a copy of our full Clinical Study Summary, please contact your Territory Manager.

AEROBIKA® OPEP Device
Oscillating Positive Expiratory Pressure or OPEP therapy helps stent open and clear blocked airways. On exhalation the device creates a unique oscillation and pressure dynamic to address the structural and functional challenges in the airways. The combination of oscillations and pressure opens the airways and aids in moving mucus into the central airways, where it can be coughed out. The device is drug-free so there are no side effects or drug interactions and it is easy-to-use, so patients are able to transition home with the device.
Clinical Resources
A quick view of the most recent peer-reviewed evidence supporting the chambers. Learn more.
- 28% decrease in readmissions for COPD exacerbations. Learn more.
- 39% decrease in all-cause rehospitalizations and lower costs for post-operative recovery. Learn more.
Contact your Territory Manager today for a copy of our full Clinical Study Summary for the AEROBIKA® OPEP device.